History
Kine sciences will always be a company that
remains in the minds of customers without losing its original intentions.
Kinescicences History
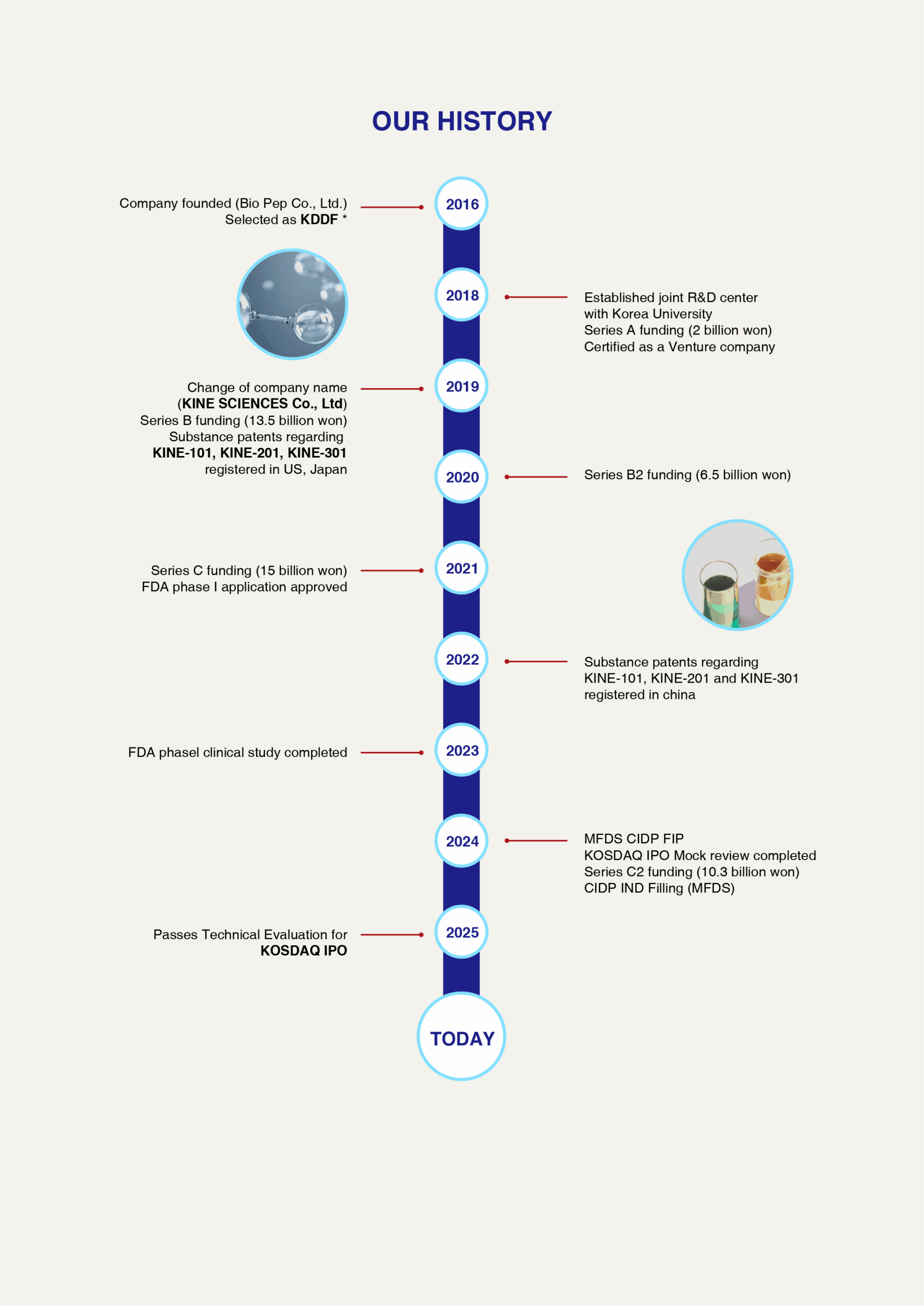
Kinesciences History
| Year | Month | Event |
|---|---|---|
| 2024 | ||
| 12 |
|
|
| 11 |
|
|
| 10 |
|
|
| 08 |
|
|
| 07 |
|
|
| 06 |
|
|
| 05 |
|
|
| 04 |
|
|
| 03 |
|
|
| 2023 | ||
| 11 |
|
|
| 10 |
|
|
| 2022 | ||
| 03 |
|
|
| 01 |
|
|
| 2021 | ||
| 12 |
|
|
| 10 |
|
|
| 09 |
|
|
| 06 |
|
|
| 05 |
|
|
| 2020 | ||
| 07 |
|
|
| 2019 | ||
| 08 |
|
|
| 07 |
|
|
| 04 |
|
|
| 03 |
|
|
| 2018 | ||
| 12 |
|
|
| 11 |
|
|
| 10 |
|
|
| 2017 | ||
| 12 |
|
|
| 2016 | ||
| 12 |
|
|
| 08 |
|
|
| Year | Month | Event |
|---|---|---|
| 2020 | 07 |
|
| 2019 | 08 |
|
| 07 |
|
|
| 04 |
|
|
| 03 |
|
|
| 2018 | 12 |
|
| 11 |
|
|
| 10 |
|
|
| 2017 | 12 |
|
| 2016 | 12 |
|
| 08 |
|
KINE SCIENCES History
| Year | Month | Event |
|---|---|---|
| 2024 | 12 |
|
| 11 |
|
|
| 10 |
|
|
| 08 |
|
|
| 07 |
|
|
| 06 |
|
|
| 05 |
|
|
| 04 |
|
|
| 03 |
|
|
| 2023 | 11 |
|
| 10 |
|
|
| 2022 | 03 |
|
| 01 |
|
|
| 2021 | 12 |
|
| 10 |
|
|
| 09 |
|
|
| 06 |
|
|
| 05 |
|
| Year | Month | Event |
|---|---|---|
| 2020 | 07 |
|
| 2019 | 08 |
|
| 07 |
|
|
| 04 |
|
|
| 03 |
|
|
| 2018 | 12 |
|
| 11 |
|
|
| 10 |
|
|
| 2017 | 12 |
|
| 2016 | 12 |
|
| 08 |
|
2024
12 : KINE-101C orphan drug designation in development stage by the Korean MFDS
11 : Partnership meeting at Bio-Europe Fall 2024
10 : Signed a material transfer agreement (MTA) with Alteogen for development of subcutaneous formulation of peptide drug
08 : KINE-101C First patient administration in Korean patient clinical trial for CIDP
07 : R&D Center integrated and relocated to Pangyo (Name: Central Research Institute)
Signed a material transfer agreement (MTA) with Swiss biotech company Biolingus for the development of oral dosage forms
Collaboration agreement with Oncocross for AI-based new drug development
(Discovering optimal new drug candidates and exploring indications based on AI platform)
KINE-101C IND Approved by Korea MFDS
KINE-201C GLP study initiated
06 : Inauguration of a new Executive Vice President, Sangyong Song M.D. & Ph.D.
Partnership meeting at Bio-US 2024
Collaboration agreement with NeuroLynx for KINE-501 & Brain disease treatment
05 : Partnership meeting at Bio-Korea 2024
04 : KINE-101C IND filing to Korea MFDS
Selection of 2024 The Ministry of Health and Welfare Grant for dementia treatment and prevention
(Developing the peptide drug targeting microglia for Alzheimer’s disease treatment), 2.25M USD
Selection of 2024 Korea Intellectual Property Protection Agency Patent Dispute Response Strategy Support Project (Patent Dispute Response Strategy Support Project (24-P00113))
03 : Partnership meeting at Bio-Europe Spring 2024
Series C2 funding (7.4 M USD)
Selection of 2024 KISTA Korea Intellectual Property Strategy Agency IP-R&D Strategy Support Project (Immune peptide new drug development technology)
2023
11 : Research Collaboration Agreement with IMMUNIQUE
Patent for material (analogue) of KINE-301 granted in Japan and Canada
10 : KINE-101A Completed Phase1 Clinical trial in US
2022
03 : CEOs of Hee Kyung Kim & Dae Ho Cho inaugurated
01 : KINE-101B GLP study initiated
2021
12 : Move the head office to PNM Tower
10 : Series C funding (10.5 M USD)
09 : KINE-101A Phase 1 IND approved by US FDA
06 : KINE-101A Phase 1 IND filing to US FDA
05 : Substance patents regarding KINE-101,
KINE-201 and KINE-301 registered in US
2020
07 : Project awarded by Korea Patent Strategy Development Institute
Series B2 funding (4.6 M USD)
2019
08 : Corporate R&D Center certification acquisition
07 : KINE-101A GLP study initiated
04 : Series B funding (9.7 M USD)
03 : Change of corporate name to KINE SCIENCES Co.,Ltd.
R&D Center of KINE SCIENCES established
2018
12 : Certified by Korea Government as a Venture Company
11 : Series A funding (1.4 M USD)
10 : Established joint research institute with Korea University
2017
12 : Patents regarding KINE-101, KINE-201 and KINE-301 filed in
major foreign countries
2016
12 : Selected as a new drug development project by the Ministry of
New Drug Development Project
08 : Company founded(Bio Pep Co., Ltd.)
